NEW PRODUCT
ARES™ X20+ Fill & Finish System
 Compact, High-Throughput Fill & Finish
Compact, High-Throughput Fill & Finish
 Cooling & Agitation
Cooling & Agitation
 Fills Bags & Vials Within Single Batch
Fills Bags & Vials Within Single Batch
 Closed System, Benchtop Design
Closed System, Benchtop Design
 Compact, High-Throughput Fill & Finish
Compact, High-Throughput Fill & Finish
 Cooling & Agitation
Cooling & Agitation
 Fills Bags & Vials Within Single Batch
Fills Bags & Vials Within Single Batch
 Closed System, Benchtop Design
Closed System, Benchtop Design
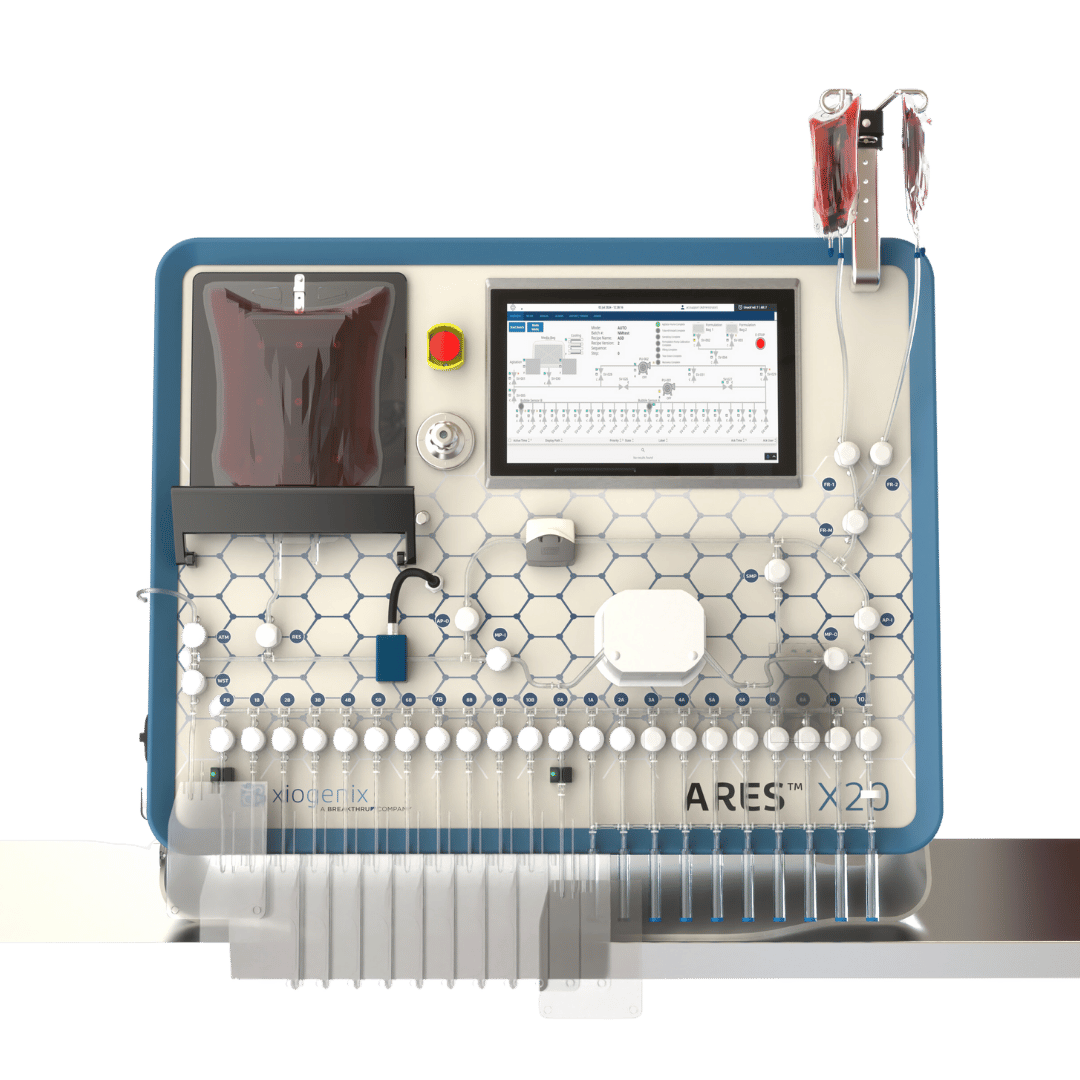
ARES™ X20+
Advanced Features

Automated Cooling & Agitation
Ensures precise temperature control and homogenization of the source material.

Formulation Flexibility
Weld up to two different media materials, such as cryoprotectant or buffer solution, to automate formulation into the source bag.
Air Evacuation
Minimizes residual air by removing air before the filling sequence.

Efficient Serial Filling
Fills one container at a time across 20 slots, with an extended configuration option to increase capacity beyond 20 containers.

Fast Filling Process
Designed to minimize cryoprotectant contact time by increasing the efficiency of the formulation and final filling processes, capable of completing the entire process in under 5 minutes.

Recoverable Prime Volume
Automated purging feature ensures that all fluid within the lines (approx. 17ml) is recoverable.
Air Management
Semi-automated modes for removing excess air and burping bags post-fill. Automated and semiautomated options for removing air before and after a filling sequence.
ARES™ X20+
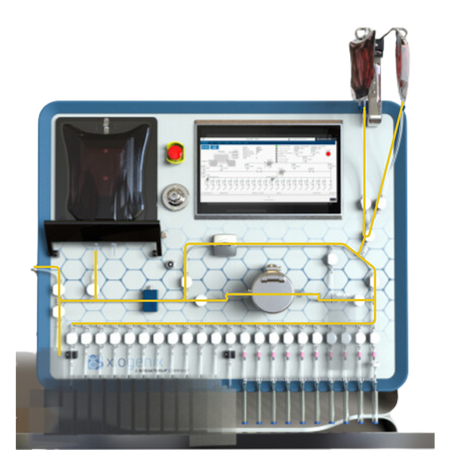
Process Flow

Weld Source Material and Formulation Media:
Securely weld the source material and formulation media to the manifold.

Setup the Manifold:
Attach the manifold to the system.

Cooling & Agitation:
Mixing of the source bag to ensure homogeneity while simultaneously cooling the bag.

Automated Formulation:
The system automates the formulation of media into the source material.

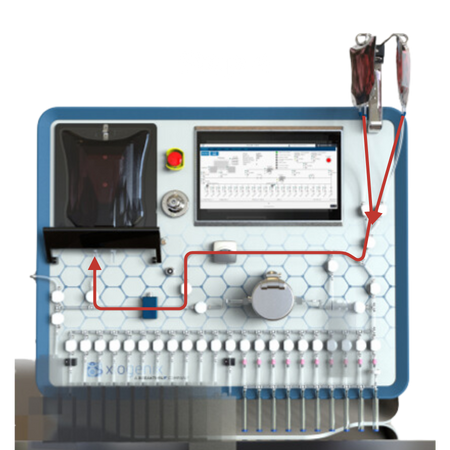
Air Evacuation:
The system has the ability to remove air from bags in an automated fashion for the distribution into the FDP containers.

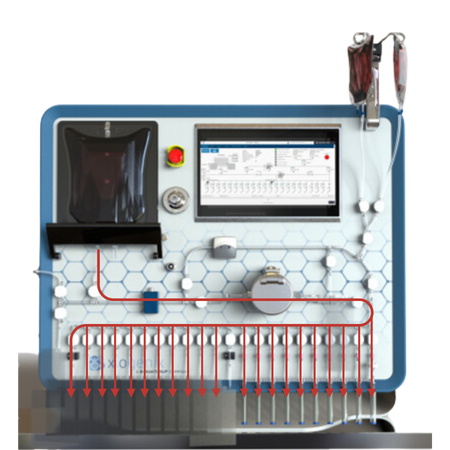
Fill Sequence:
The source material is serially distributed into the FDP containers.

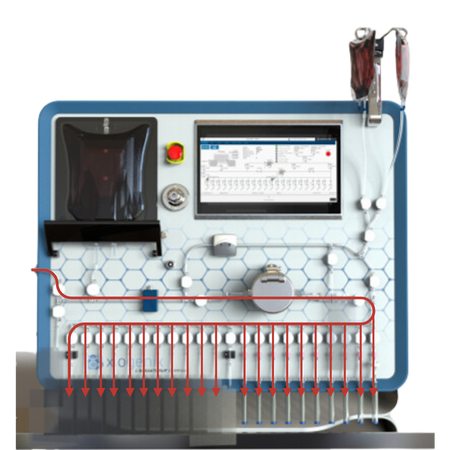
Post Fill Purge Sequence:
The system automatically purges the tubing to recover product, minimizing waste.

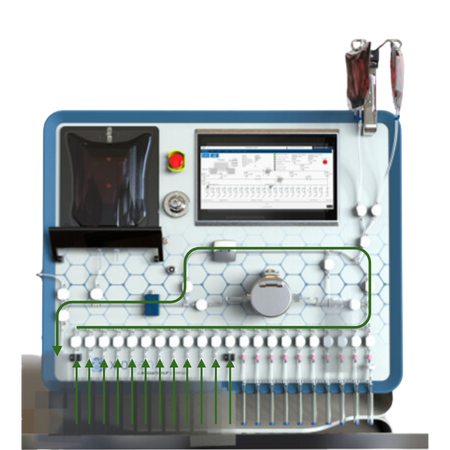
Post Fill Air Management:
If required, system has the capability to remove air following the fill sequence.



Connect with our Cell & Gene Therapy Team

Josh Jendusa
Director of Programs
"Success is not final, failure is not fatal: It is the courage to continue that counts." ~ Winston Churchill

Allie Schroeder
Director of Sales & Marketing

Disrupt. Create. Advance.® — Engineering Solutions for the Pharmaceutical Industry.
CONNECT WITH US
IMPORTANT LINKS
About
Cell & Gene Therapy
Tissue Banking
Services
News
Connect
Careers
LOCATIONS
United States
W160 S6550 Commerce Drive
Muskego, WI 53150
European Demonstration Office
46 Energieweg
Nijmegen 6441CX NL
Copyright 2025 Xiogenix. All rights reserved.