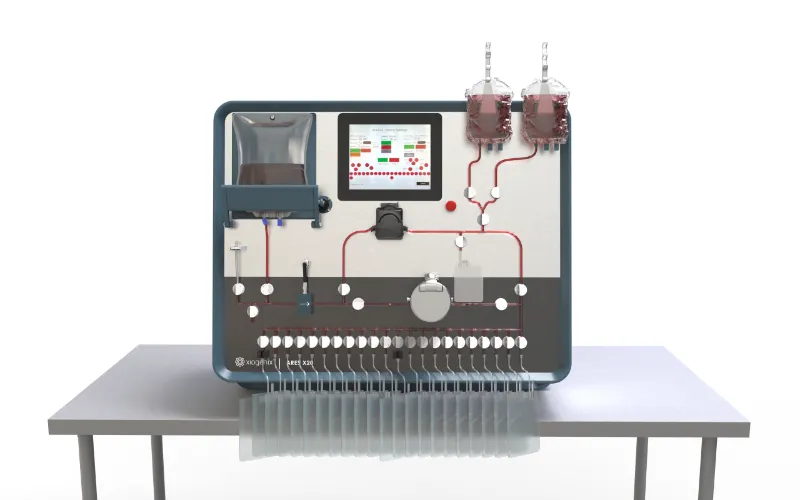
Advanced Cell Therapy Studies with Ares™️ X20
At Xiogenix, we are committed to advancing the field of cell therapy through innovative solutions. Our new Ares™️ X20 Formulation, Fill & Finish System is at the forefront of precision and efficiency, designed to meet the unique needs of the cell therapy market. We are currently conducting studies with the following cell lines, demonstrating Ares™️ X20’s capabilities in optimizing cell therapy workflows.

TIL (Tumor-Infiltrating Lymphocytes)
TIL therapy involves extracting immune cells from a patient’s tumor, expanding them in the lab, and reinfusing them to enhance the body’s natural ability to fight cancer. By amplifying these tumor-targeting cells, TIL therapy has shown promise in treating advanced cancers, including melanoma and other solid tumors. TIL therapies are seen to have varying environmental sensitivities compared to traditional CAR-T therapies, making process adjustments on the Ares device critical for ensuring the highest product quality and efficacy. Our studies using Ares™️ X20 focus on maintaining the potency and integrity of TILs during the critical fill and finish stages, ensuring the highest quality for clinical applications.
CAR-T (Chimeric Antigen Receptor T-cells)
CAR-T cell therapy is a personalized cancer treatment that modifies a patient’s T-cells to recognize and attack specific markers present on malignant B cells. This therapy has revolutionized treatment options for blood cancers like leukemia, lymphoma, and multiple myeloma. Today, CAR-T therapies are even being used to treat diseases beyond oncology, with large developments in autoimmune disease treatment. Our Ares™️ X20 device supports the precise and sterile handling of CAR-T cells during the fill and finish process, which is critical to the success of this complex and life-saving therapy. With CAR-T studies, Ares™️ X20 is helping to ensure optimal cell viability through filling efficiency and robust parameter control.
iPSC/HSC (Induced Pluripotent Stem Cells/Hematopoietic Stem Cells)
Stem cell therapies offer an innovative approach to regenerative medicine and immunotherapy by providing off-the-shelf, allogeneic cell therapies. These therapies are derived from iPSCs and HSCs, which can be programmed to differentiate into specific cell types, including hematopoietic stem cells (HSCs) for blood-related disorders or cancers. Our studies focus on using Ares™️ X20 to manage the production of iPSC/HSC-derived cells, ensuring a smooth and sterile fill and finish process that supports large-scale manufacturing for future clinical deployment. Our system can adapt to the varying cell concentrations seen with these therapies to ensure optimal cell distribution and process flow to achieve favorable cell viability.
NK Cells (Natural Killer Cells)
Natural Killer (NK) cell therapies are a specified cell line that also offers an innovative approach to immunotherapy by providing off-the-shelf, allogeneic cell therapies. These therapies can commonly be derived from iPSCs and other stem cell sources, which can be programmed to differentiate into natural killer (NK) cells. NK cells play a crucial role in targeting and killing tumor cells without prior sensitization, offering potential advantages for cancer immunotherapy. Our studies focus on using Ares™️ X20 to manage the downstream aliquoting of NK-based cell therapies, ensuring a smooth and sterile fill and finish process that supports large-scale manufacturing for future clinical deployment. Our system is designed to adapt to varying cell line characteristics, ensuring optimal cell distribution and process flow to achieve high cell viability and therapeutic efficacy with NK products.
Why Ares™️ X20?
The Ares™️ X20 is specifically designed to meet the high demands of cell therapy fill & finish processes, ensuring:
- Precision in handling sensitive cell lines
- Sterility for regulatory compliance and patient safety
- Scalability to meet growing demand in cell therapy production
- Automation to streamline workflows and reduce manual intervention
By using Ares™️ X20, we are advancing the efficiency and safety of cell therapy manufacturing, enabling more reliable and consistent treatments for patients in need.
Connect with our Cell & Gene Therapy Team

Sami Reimer
Director - Cell & Gene Therapy
"The way to get started is to quit talking and begin doing." ~ Walt Disney

Josh Jendusa
Product Manager
"Success is not final, failure is not fatal: It is the courage to continue that counts." ~ Winston Churchill

Disrupt. Create. Advance.® — Engineering Solutions for the Pharmaceutical Industry.
CONNECT WITH US
IMPORTANT LINKS
About
Cell & Gene Therapy
Tissue Banking
Services
News
Connect
Careers
LOCATIONS
UNITED STATES
W160 S6550 Commerce Drive
Muskego, WI 53150
EUROPE
46 Energieweg
Nijmegen 6441CX NL
Copyright 2025 Xiogenix. All rights reserved.
